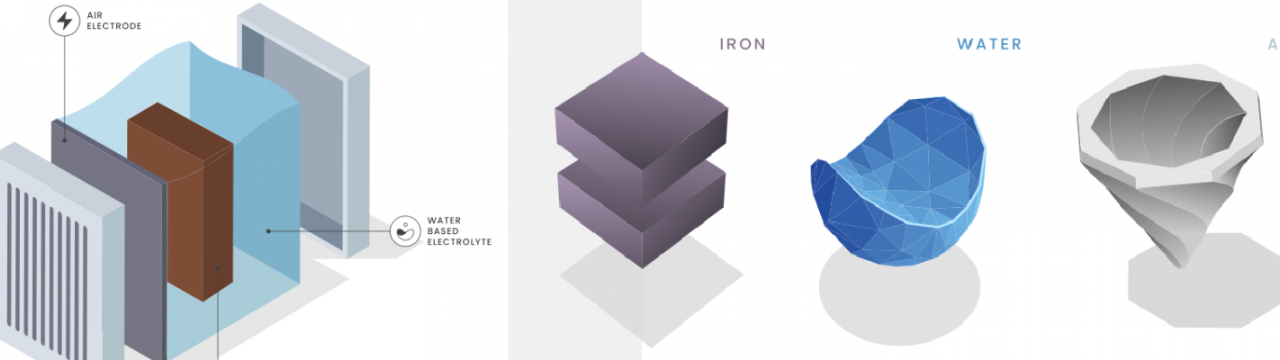
Background and problem statement
Efficient energy storage is vital for renewables. Iron-air batteries offer a promising, low-cost alternative with 100+ hours of storage but are hindered by high overpotentials due to sluggish Oxygen Evolution and Reduction Reactions (OER/ORR). The primary challenge for iron-air batteries lies in the slow kinetics of OER and ORR, which cause high overpotentials and reduce overall system efficiency. Current electrocatalyst materials exhibit insufficient activity, degrade, or suffer from poor electrical conductivity and weak catalyst-substrate interactions. Overcoming these barriers requires advances in catalyst composition, microstructure engineering, and manufacturing to boost active surface area, electrochemical reaction kinetics.
Project objective
The goal of this project is to develop and optimize advanced electrocatalyst materials within two years to enhance reaction efficiency, reduce energy losses, and improve the round-trip efficiency. By tailoring the composition, structure, and catalyst-substrate interactions, the research aims to achieve higher catalytic activity, lower overpotentials, and greater long-term stability, ultimately contributing to more efficient and sustainable energy storage solutions. This goal directly addresses the key bottleneck limiting the performance of iron-air batteries - high overpotential - and contributes to increasing their commercial viability.
Project results
This project will lead to the development of advanced electrocatalysts, such as optimized nickel-iron and manganese-oxide catalysts, engineered to enhance reaction efficiency at the cathode by significantly reducing overpotentials in both the ORR and OER compared to the currently used cathodes and electrodes in the Ore Energy battery. As a result, the electrochemical efficiency will be increased, with a higher round-trip efficiency, bringing Ore Energy’s iron-air battery close to metrics observed in other long-life battery solutions, like Ni-Fe systems, but carrying a much higher power density.