SPECT camera calibration and 225Ac quantification
Quantitative SPECT imaging of 225Ac is technically challenging because this promising isotope for targeted alpha therapies lacks suitable direct gamma emission. As a result, imaging relies on measurement of daughter isotopes. Due to its complex decay scheme and quantification, there is currently no consensus on the best way to image 225Ac with high accuracy. In this WP, we will evaluate the quantification accuracy using calibration sources traceable to the primary standard. Our work will focus on optimizing image acquisition settings. In addition, we will perform longitudinal SPECT phantom scans in preclinical settings.
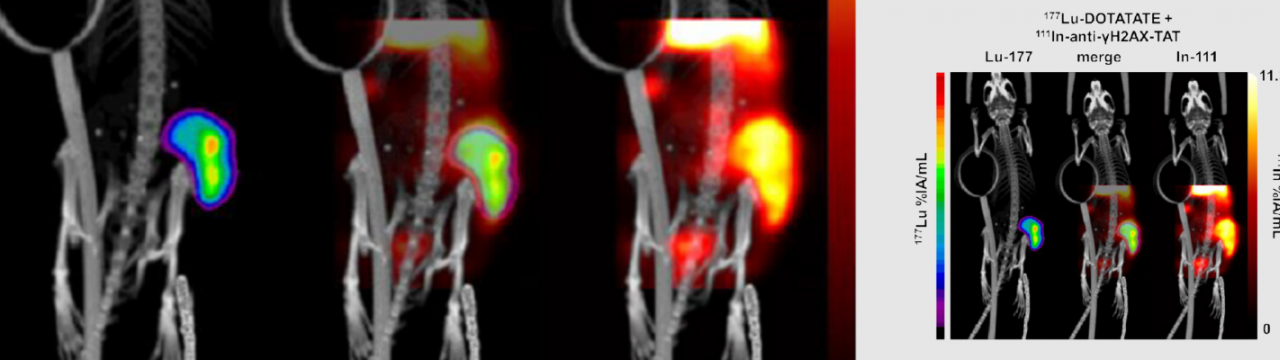
Dual isotope imaging using in vivo biodosimeters
The techniques we will develop here will enable real-time assessment of tumour responses to radionuclide therapy by visualizing DNA damage in cancer tissues. By tracking DNA damage markers, we will be able to predict the effectiveness of treatments like 177Lu or 225Ac, enhancing personalized treatment plans. It will also allow to understand the effects of tumour heterogeneity (intratumoural differences in TRT target expression, delivery, and effect).